Lipopolysaccharide transport
Gram-negative bacterial infections are a significant threat to human health due to increasing resistance to all classes of clinically-used antibiotics. Gram-negative bacteria are extremely challenging to kill because of their unique asymmetric outer membrane with lipopolysaccharide (LPS) in the outer leaftlet. LPS is a large, highly-charged glycolipid, and lateral interactions between LPS molecules and Mg(II) ions create a dense electrolyte mesh that prevents entry of hydrophobic molecules including most antibiotics. To maintain this protective barrier, cells must quickly and accurately transport LPS to the cell surface. Minor disruptions to LPS transport increase membrane permeability and cause antibiotic susceptibility, and major disruptions cause cell death. Thus, the LPS transport machinery is an attractive target for antibiotic development.
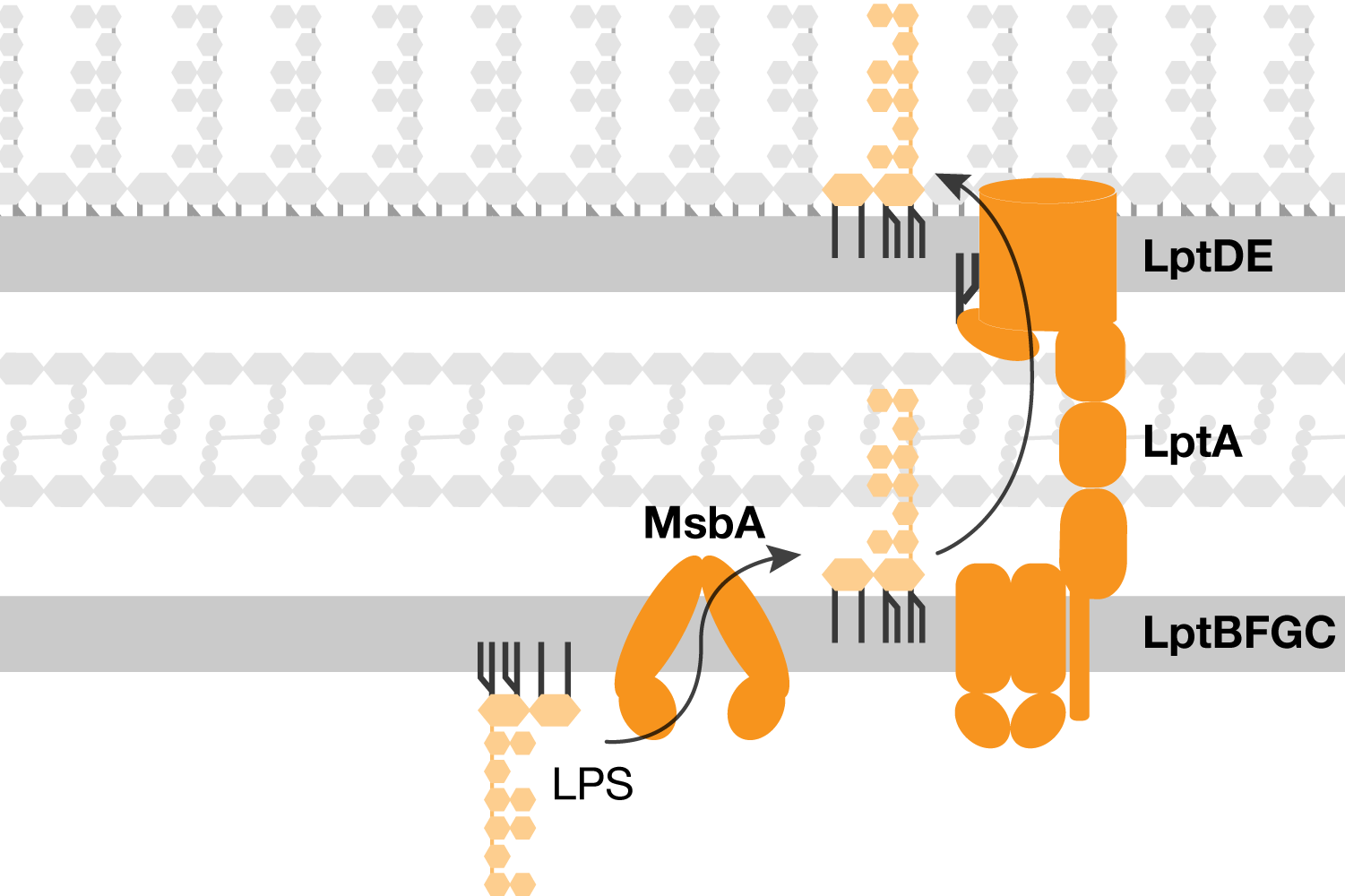
Lipopolysaccharide transport overview
Lipopolysaccharide (LPS) is first synthesized in the cytoplasm and then is transported across the aqueous periplasm and through the outer membrane via a protein bridge. Our lab studies the mechanism of LPS transport to understand how to inhibit outer membrane biogenesis.
Millions of lipopolysaccharide molecules are transported to the cell surface against a concentration gradient each time the cell divides. The cell must maintain efficient, unidirectional transport of lipopolysaccharide to allow bacterial growth. Additionally, most bacteria produce many structural variants of lipopolysaccharide and the transport machinery must be capable of handling these diverse cargo. We aim to answer the fundamental questions of how the lipopolysaccharide transport machinery overcomes these challenge in order to understand how to target the lipopolysaccharide transport to develop new antibiotics.
Banner image: Accumulation of subcellular lipopolysaccharide in E. coli upon MsbA inhibition, visualized with a synthetic fluorescent polymyxin derivative (Moison et al. ACS Chemical Biology 2017)
Selected PUBLICATIONS
Novobiocin enhances polymyxin activity by stimulating lipopolysaccharide transport. M.D. Mandler, V. Baidin, J. Lee, K.S. Pahil, T.W. Owens, D. Kahne. J Am Chem Soc 2018;140:6749-53. PubMed
Cell-based screen for discovering lipopolysaccharide biogenesis inhibitors. G. Zhang, V. Baidin, K. Pahil, E. Moison, D. Tomasek, N.S. Ramadoss, A.K. Chatterjee, C.W. McNamara, T.S. Young, P.G. Schultz, T.C. Meredith, D. Kahne. Proc Natl Acad Sci USA 2018;115:6834-9. PubMed
Lipopolysaccharide is transported to the cell surface by a membrane-to-membrane protein bridge. D.J. Sherman, R. Xie, R.J. Taylor, A.H. George, S. Okuda, P.J. Foster, D.J. Needleman, D. Kahne. Science 2018; 359:798-801. PubMed
The antibiotic novobiocin binds and activates the ATPase that powers lipopolysaccharide transport. J.M. May, T.W. Owens, M.D. Mandler, B.W. Simpson, M.B. Lazarus, D.J. Sherman, R.M. Davis, S. Okuda, W. Massefski, N. Ruiz, D. Kahne. J Am Chem Soc 2017;139:17221-4. PubMed
Cytoplasmic ATP hydrolysis powers transport of lipopolysaccharide across the periplasm in E. Coli. S. Okuda, E. Freinkman, D. Kahne. Science 2012; 338:1214-7. PubMed